Functional Ultrasound Microscopy: Sensing the Whole Brain Neuronal Activity at the Micron Scale
Ultrasound scanners enable the observation of organs through the skin, and as such are widely-used in medical imaging, mostly in obstetrics or in cardiology. The use of ultrasound for neuroimaging remains so far limited to detecting large (several millimeter in diameter) blood vessels in the brain. There is however a major interest in looking more precisely at the cerebral vasculature, as many neurodegenerative diseases – Alzheimer, dementia – involve dysfunctions of the small cerebral vessels.
Neurovascular coupling: a dialogue between neural and vascular networks
A tight interaction occurs between the brain vascular and neuronal activities: blood vessels supply neurons in oxygen and nutrients, and therefore support the neuronal activity. This essential interaction, called neurovascular coupling, is exploited in neuroimaging: detecting blood flow variations allows to detect brain activation.
Functional ultrasound (fUS) does so with a very high sensitivity: it is capable of detecting very subtle changes in the cerebral blood volume. And it provides imaging performances inherent to the use of ultrasound waves, i.e. a large field of view covering the entire brain, and a resolution of several hundreds of microns.
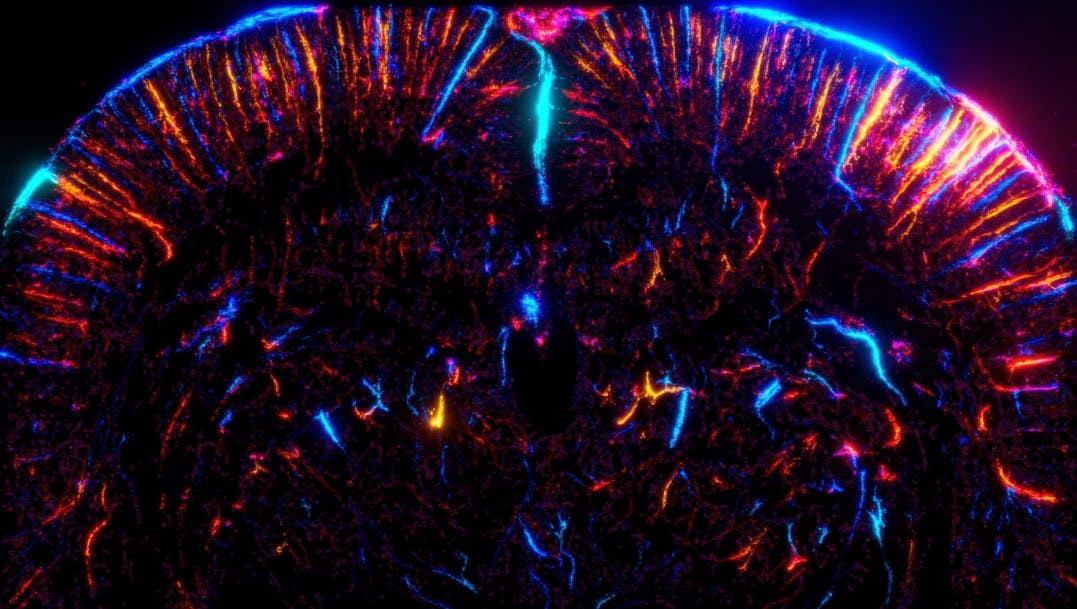
To distinguish smaller vessels, researchers of the institute Physics for Medicine Paris have developed a technique called ultrasound localization microscopy (ULM), achieving a much better spatial resolution than standard ultrasound. Let’s imagine that you try to capture a satellite view of a narrow road. It might be challenging to distinguish a narrow path from afar. Now, if a motorbike travels on that road with its headlight on, you might see its halo and the center of the halo will inform you accurately on the motorbike’s position, and therefore reveal the narrow road’s position. Localization microscopy relies on that same principle: a bright, point-like object can be localized accurately by pinpointing the center of its halo. In ULM, the “bright” objects are biocompatible micron-size bubbles, injected in the blood circulation. Tracking these micron-size bubbles as they travel in the blood stream reveals the location of blood vessels with a micron-size accuracy, and across large fields of view using ultrasound imaging. By accumulating the tracks of millions of microbubbles, the researchers have reconstructed unique pictures of the microvasculature anatomy at the whole-brain scale in rodents and in human patients, as published in previous studies (Demené et al., Science Translational Medicine 2017; Demeulenaere et al., eBioMedicine 2022). However, this technique is not fast and sensitive enough to catch dynamically the local changes of blood flow induced by the neuronal activity.
Non-invasively probing brain activity at the microscopic level
In the current study, published in Nature Methods, the researchers solve this problem and go much further in the analysis of the microbubbles’ journey across the brain vasculature of a rat. Not only do they image the brain microvasculature, but they also detect the local brain activation by calculating the number and speed of microbubbles passing in each vessel: when a region of the rat brain activates, the blood volume increases locally as a result of the neurovascular coupling, dilating the vessels and allowing more microbubbles to pass. In other words, it becomes possible to extract dynamic information from ULM data. Tracking microbubbles does not serve only to localize micro-vessels, but it also enables to sense the functional activity at the micro-vessel scale. All the technical developments yield a new neuroimaging method, coined functional ultrasound localization microscopy (fULM), with a unique combination of three major advantages – non-invasive quantification of the brain activity, at microscopic resolution, and across the whole-organ scale – integrated in an easy-to-use and cost-efficient ultrasound scanner.
Demonstrating the concept of fULM is a pivotal scientific leap, but yet it is only the first step into a new and vast research area in neuroscience. Each one of the millions of microbubbles captured during a fULM acquisition carries lots of biologically-relevant information to be explored. The upcoming tasks for the scientists will be to exploit, process and relate this enormous amount of information to the mechanisms involved in cerebro-vascular and neurodegenerative diseases.
Full Publication
The team is supported by the AXA Research Fund through the AXA Chair in Biomedical Ultrasound.
Video information
Credits: Alexandre Dizeux / Physics for Medicine Paris
August 2022
Discover research projects related to the topic
Mental Health & Neurology
Extreme Weather Events
Pollution
Alzheimer's Disease, Dementia & Neurodegenerative Diseases
Droughts & Heatwaves
Air Quality
Mécénat des Mutuelle
France
CLIMABRAIN: Impacts of Extreme Weather on the Most Vulnerable Living with Alzheimer's disease
In the context of the increase in extreme weather events due to climate change, the project led by Tarik Benmarhnia... Read more

Tarik
BENMARHNIA

